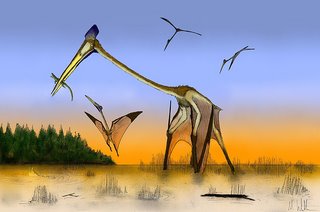
People often talk of getting a ‘culture shock’ when they travel abroad. But in the world of zoological uber-nerdiness, you don’t need to go abroad to experience a culture shock, you merely need to be an exposed to an idea that is shockingly alien and counter-intuitive. I still have fond memories of those days back in 1997 when I first visited the School of Earth & Environmental Sciences at the University of Portsmouth to be interviewed by the man who would later become my phd supervisor, Dave Martill. Of all the surprising things I was exposed to at the time, none was more striking and bizarre than the gigantic wall-mounted display on azhdarchid pterosaurs. Featuring a life-sized wing skeleton, some photos of a grinning German man* holding a bone, and a giant colour mural by John Sibbick, it’s a pretty good exhibition, and eight years later it’s still in the same place.
* Dino Frey, guru of ‘konstruction morphology’
Based on various of their morphological details, I had concluded that azhdarchids were most likely stork-like generalists that made their living by picking up assorted invertebrates and vertebrates, terrestrial and aquatic. Several other workers had also expressed a preference for this hypothesis. But Sibbick’s giant colour mural depicted azhdarchids in an altogether different manner: they were shown wheeling above a vast expanse of ocean, gliding above the water surface and swooping down to grab fish. They were depicted as ‘mega-skimmers’, and Dave was later to explain to me why he and his colleagues favoured this skim-feeding hypothesis. I found it unconvincing and told him about the merits of the stork idea. He disagreed. We still disagree.
Today we have a new pterosaur worker in our research group, Mark Witton, and he’s been looking at the morphology and palaeobiology of azhdarchids, among other things. At the risk of stealing his thunder (sorry Mark), I will say that he, also, is a supporter of the stork-like model. If you combine this with the recent work Dave and I have been doing on the non-azhdarchid azhdarchoids
Tupuxuara and
Thalassodromeus (the latter of which was claimed to be a skim-feeder), you can understand why the topics of skim feeding and azhdarchid lifestyles have become much discussed within our research group. Mark and I are obviously in happy agreement, but we still have to turn Dave around. I’ve wanted to write up my thoughts on this area for a while now, so here we go.
Interpreting azhdarchid palaeobiologySince the announcement of its discovery in 1975,
Quetzalcoatlus – the best known and best studied azhdarchid – has been imagined in several different ways. Initially it was interpreted as a vulture-like scavenger that soared over the Late Cretaceous landscape in search of dinosaur carcasses. Why was it interpreted this way? Well, apparently because
Quetzalcoatlus was a big flying animal found in the same deposits as dinosaur bones (Lawson 1975). From a scientific perspective that’s not exactly compelling.
A second hypothesis was proposed by Langston (1981): that azhdarchids fed on burrowing invertebrates by probing for them in the substrate. This was also adopted by Wellnhofer (1991) (see p. 145, where Wellnhofer states ‘All this allows the possibililty that
Quetzalcoatlus used its slender, pointed beak to search
in the ground [my emphasis] for the molluscs and crabs that lived in the shallow pools of water’). Like Lawson’s scavenging idea, this appeared to be based on nothing more than circumstantial association of
Quetzalcoatlus with other fossils, in this case invertebrate burrows. The same idea was endorsed by Lehman & Langston (1996), though only in an abstract. Lehman & Langston (1996) later came under extremely heavy fire from some workers, but the fact that Langston had actually proposed the probing idea first, and that Wellnhofer had agreed with it, seems to have been missed.
Describing the Asian azhdarchid
Azhdarcho, Nesov (1984) was the first to propose a radically different hypothesis: that these pterosaurs might have behaved like
Rynchops, the skimmers. These charadriiform birds fly low over the water surface, trawling their unusual, laterally compressed lower jaws through the water, snapping up the fish and crustaceans that they make contact with. However, Nesov didn’t base this idea on any features he observed among members of Azhdarchidae: he assumed that azhdarchids might have behaved this way, simply because other workers had earlier proposed a skimming lifestyle for
other Cretaceous pterosaur groups. He wrote ‘If it is assumed that the Azhdarchinae could have flown like the Ornithocheirinae and Pteranodontinae – that is, like the Recent skimmers…’ (p. 42). He went on to propose that azhdarchids might have been swimmers (that’s not a typo) that captured aquatic prey, or that they might have ‘been able to hunt poorly flying vertebrates in the air’. Neither latter idea seems reasonable.
Paul (1987a, b) seems to have been the first to reject the scavenging hypothesis, at least in print, stating of
Quetzalcoatlus that ‘Its slender, two-meter beak, with only thin bars around the external nares, is too weak for regular scavenging’ (p. 20). Even better, he suggested a fourth possible lifestyle, proposing (Paul 1987a) that
Quetzalcoatlus ‘probably patrolled water courses, like a three-meter-tall stork, picking up fish and small animals’. I found Paul’s argument moderately compelling when I first read it I-don’t-know-how-many-years-ago, and I still find it moderately compelling today. Padian (1988) also rejected the scavenging idea and regarded azhdarchids as heron-like, noting that 'Langston, who knows [
Quetzalcoatlus] better than anyone, finds some suggestive resemblances to a heron or egret' (p. 64). Does this mean that Langston had given up on the probing idea? Noting the long, inflexible azhdarchid neck, Halstead (1989) wrote that ‘
Quetzalcoatlus had a neck which could only move up and down and was specialized for dipping down into the water to snatch fish’ (p. 160). This could perhaps be construed as agreement with the stork-like model.
In an article devoted exclusively to azhdarchid lifestyle, Iñaki Rodriguez Prieto (1998) argued against the scavenging hypothesis and agreed with Nesov’s skimming idea. Morphological features were used to support the latter concept, but they were either erroneous or just incorrect. Prieto argued that the bill of
Quetzalcoatlus was laterally compressed, that terrestrial abilities were very poor, and that the uropatagia would have made the hind limbs functionally similar to the forked tails seen in highly aerial birds like frigate birds, swallows, and some kites. By implication,
Quetzalcoatlus was deemed specialized for feeding on the wing, and it was on this basis that Prieto chose to follow the skimming hypothesis. All the features cited by Prieto are problematical, as we’ll see later. Prieto’s article was in Spanish and has been mostly overlooked.
Kellner & Langston (1996) also favoured the skimming hypothesis but did the same thing that Nesov did: they regarded it as ‘at least plausible’ for
Quetzalcoatlus on the basis of the fact that it had been ‘previously advocated for
Rhamphorhynchus … and later assumed for many other pterosaurs, including the larger toothless pterosaurs’ (p. 231). In other words, they didn’t provide any supporting evidence at all, but merely elected to follow conclusions made for other, morphologically different pterosaur taxa (and, by the way, the concept of skim-feeding in rhamphorhynchids and those other pterosaurs isn’t necessarily any more secure than it is for azhdarchids).
Unwin
et al. (1997) noted that ‘unpublished functional studies and the circumstances of their preservation suggest that [azhdarchids] may have been piscivorous … feeding from the water surface while on the wing and using the long neck as a ‘fishing rod’’ (p. 48). They cited an abstract and Unwin’s unpublished thesis when discussing this area, but didn’t elaborate as to why they favoured the skimming hypothesis. Martill (1997), while being harshly critical of Lehman & Langston’s mud-probing idea, also agreed with the skimming hypothesis, but, I would argue (sorry Dave), presented it as a just-so story, not as an evidence-led hypothesis. The presence of a ‘highly streamlined skull’ (p. 73) was used to support skimming behaviour, as was the long stiff neck. Martill
et al. (1998) rejected both the scavenging and mud-probing hypotheses, and regarded azhdarchids as ‘aerial piscivores or planktivores’ (p. 57). While noting the inflexibility of the neck, they didn’t provide any supporting evidence for this hypothesis however.
Finally, Bennett (2001) noted that the femora and metatarsal V of
Quetzalcoatlus were robust relative to those of other pterodactyloids, and that its feet were larger and more robust than those of ornithocheiroids. These observations led him to regard
Quetzalcoatlus as ‘better suited to terrestrial locomotion than
Pteranodon’, and specialised for feeding on the ground. He wrote that ‘
Quetzalcoatlus remains have been found far inland where they seem to have been heron- or stork-like in their ecology despite their great size’ (p. 136).
So that’s what’s in the literature: four hypotheses (scavenger, skimmer, stork and mud-prober). It’s problematical that hardly any morphological features have ever been used to support them, and in fact some of them seem decidedly arm-wavy and intuitive, rather than evidence-led. So in the interests of introducing some hypothesis testing into this area, I’ve listed below all those hypotheses that I consider at least feasible, and have made predictions as to what evidence we would require in order to consider them reasonable. I do not consider Nesov’s ideas that azhdarchids were swimming predators, or that they routinely captured flying vertebrates (presumably other pterosaurs and birds) likely, nor have I considered lifestyles that are obviously discordant with azhdarchid morphology (e.g. that they were deep divers, plunge divers, herbivores, aerial pirates or filter-feeders). I looked at as much literature as I could on form and function in bird bills, and also examined relevant specimens.
The hypothesesHypothesis 1: azhdarchids were vulture- or marabou-like scavengers, soaring over land and feeding from the carcasses of large terrestrial vertebrates.
Predictions: proficient terrestrial abilities; bill and head capable of probing into body cavities; bill robust around narial openings; flexible neck; highly developed soaring or gliding skills. Note that hooked bill tips are not necessarily needed for this lifestyle, given that marabou storks and some corvids (e.g. Thick-billed raven
Corvus crassirostris) routinely scavenge, yet have pointed bill tips. Witmer & Rose (1991) noted this.
Hypothesis 2: azhdarchids were mud-probers (like sandpipers), pushing their bills into sediment in search of burrowing prey.
Predictions: proficient wading abilities (indicating by long, spreading toes); no need for highly developed soaring or gliding skills; skull should be specialized for probing, either with features allowing powerful gaping, with adaptations allowing rhynchokinesis, with a cross-section recalling that seen in mud-probing birds, and/or with well-developed tactile organs at the bill tip (e.g. Herbst corpuscles); neck should be reasonably flexible. Birds that probe sediment in search of prey have been shown to rely either on touch, on the detection of vibrations produced by the prey, or on the detection of pressure gradients surrounding hard objects (Gerritsen & Meijboom 1986, Piersma
et al. 1998, Nebel
et al. 2005). Pressure-sensitive organs termed Herbst corpuscles, embedded within pits on the premaxillary and dentary tips, are closely packed and particularly numerous in birds that probe sediments.
Hypothesis 3: azhdarchids were spear-fishers (like herons and anhingas), stalking fish in shallow water and spearing the body of the prey with sharp bill tips.
Predictions: proficient wading abilities (indicating by long, spreading toes) or swimming abilities; sharply pointed, spear-like bill; flexible neck that allows rapid darting of bill towards prey.
Hypothesis 4: azhdarchids were skim-feeders, flying low over the water and trawling the lower jaw through the water. In contrast to that of many other birds, the feeding behaviour and cranial morphology of skimmers has been well described (Arthur 1921, Tomkins 1951, Bock 1960, 1964, Zusi 1962).
Predictions: no need for proficient terrestrial abilities; highly skilled at fast, level flight; lower jaw laterally compressed and blade-like; streamlined bill; jaw joint, back of skull and neck able to withstand sudden jarring, with accessory articulation present between mandible and basicranium; upper jaw can be elevated relative to the basicranium (and is thus clear of the water surface during skimming); jaws capable of extremely rapid closure.
Hypothesis 5: azhdarchids were surface gleaners, or dippers, flying low over water and grasping food from the water surface (like albatrosses or frigate birds).
Predictions: no need for proficient terrestrial abilities (hind limbs may even be strongly reduced); highly developed soaring or gliding skills; jaws elongate with down-curved tips; flexible neck allowing the animal to reach down and behind itself as it picks up food while flying over the water.
Hypothesis 6: azhdarchids were stork-like generalists, picking up assorted invertebrate and vertebrate prey from shallow water and/or terrestrial environments.
Predictions: proficient terrestrial abilities; no need for highly developed soaring or gliding skills; bill elongate but lacking specializations (such as lateral or dorsoventral compression, keels, or hooked bill tips); neck flexibility not required as the neck only needs to bring the bill tips close to the ground; head-neck joint, at least, should be flexible.
The morphological evidenceLet’s now see how the morphological data matches with these hypotheses and their predictions. Firstly, despite all those early claims making out that azhdarchids were like immense vultures, with tremendously elongate wings indicative of superb soaring or gliding skills, we now know that this was just not true. In fact, their legs were proportionally long, their wings were proportionally short compared to those of other large pterosaurs, and preserved wing membranes (which reveal that the brachiopatagium attached to the ankle) show that their wing membranes actually made their wings proportionally broad, and with low aspect ratios. As Frey
et al. (2003) concluded, azhdarchids exhibited poor gliding performance compared with other large pterosaurs. Prieto’s (1998) proposal that azhdarchid legs formed a pseudo ‘forked tail’ is nonsense given that the brachiopatagia incorporated the legs into the wings: they didn’t trail behind the body as do a bird’s tail feathers.
In terms of terrestrial abilities, we should note first that pterodactyloids in general were quite capable quadrupeds, and there is little reason to regard them as clumsy or helpless when grounded. Sure, they couldn’t sprint at speeds equaling those of cursorial animals, but there is every indication that they were proficient walkers, more than capable of foraging quadrupedally on the ground or in shallow water. This is backed up by functional morphology, computer modelling, and evidence from trackways. With their proportionally short wings, long legs with robust femora, and large, robust feet (Bennett 2001), azhdarchids were likely to have been even better suited for terrestrial foraging than most other pterodactyloids. These lines of evidence suggest that azhdarchids were not specialized for a life on the wing (contra Prieto 1998): rather, they were better on the ground than were most other pterosaurs.
What does skull anatomy suggest? Good azhdarchid skulls are few and far between, with the best one being the incomplete rostrum described by Kellner & Langston (1996). In basic terms, the rostrum is shaped like a very long scalene triangle: it’s deepest at the level of the nasoantorbital fenestra, but gradually tapers rostrally to a point. Some kind of bony crest is present over the caudal part of the nasoantorbital fenestra. Ignoring the crest, which living animals have a rostrum shaped like this? Storks, and not much else. Apparently the specimen described by Kellner & Langston (1996) is squashed flat, however, which makes it impossible to confirm whether the snout had the subrounded cross-sectional shape seen in storks. This is the spanner in the works, because if the skull is strongly compressed laterally, then the skull really isn’t stork-like at all, but probably suited for some other, quite different mode of life. Like skimming.
But hold on: this isn’t the only azhdarchid skull fragment known. Firstly, there’s
Zhejiangopterus. Again, we have a pointed, elongate, overall stork-like rostrum, but again the only figured specimen is apparently squashed flat, so it’s not much use here. Aha, but there’s
Azhdarcho. Its rostrum fragments clearly belonged to a long, pointed (cough - stork-like - cough) rostrum that would have been subtriangular in cross-section, with the flat palate forming the triangle’s base (Nesov 1984). An incomplete three-dimensional rostrum from Morocco, identified as ‘?Azhdarchidae’, was described by Wellnhofer & Buffetaut (1999). It most certainly is not strongly compressed laterally, but is instead like a broad-based triangle in cross-section. Finally, a complete mandible is known for the Hungarian azhdarchid
Bakonydraco. It’s pretty odd, being pointed at its tip, slightly concave dorsally at the symphysis, and with a ventral mid-line ridge. The ridge is ventrally rounded, and not keel-like (Ősi
et al. 2005). So, again, it's not laterally compressed.
Evaluation of the hypothesesOn the basis of all these features, how do the various hypotheses hold up?
Hypothesis 1 (the idea that azhdarchids were vulture- or marabou-like scavengers) doesn’t stand up too well: while it’s been all but rejected by some workers, note that they’ve only had scavenging raptors in mind, and haven’t thought of comparing azhdarchids with marabou storks or scavenging corvids. In contrast to scavenging raptors, corvids and marabous, the azhdarchid rostrum does not appear to have been well braced around its openings (this is the naris in the birds, but the nasoantorbital fenestra in the azhdarchids), nor (with its bony dorsal crest) is the skull well suited for probing into body cavities, nor is the long, stiff neck in agreement with this lifestyle. Finally, while proficient terrestrial abilities were present, it does not seem that azhdarchids were specialized for long-distance soaring flight, as obligate scavengers are. I therefore feel that Hypothesis 1 can be rejected. In fact, even facultative scavenging like that present in marabous seems unlikely for azhdarchids, as (unlike marabous) their bills were weakly braced around the bony openings.
There are no cranial specializations consistent with Hypothesis 2 (mud-probing). Azhdarchid bill tips most certainly lack the sensory pits that house Herbst corpuscles in birds, though whether these would be present in a mud-probing pterosaur anyway is a good question. Regardless, the long, stiff azhdarchid neck doesn't match what is predicted for mud-probers either, and this hypothesis must also be rejected. Hypothesis 3 (spear-fishing) can also be rejected given that it is hard to imagine how the long, stiff neck could permit rapid lunging, stabbing and/or grabbing, plus the bill tip morphology is not spear-like as it is in the birds that practice this lifestyle.
We next come to the most popular Hypothesis: number 3, the skimming one. Despite its popularity I have to say that this is weak and not supported by the morphological evidence. While azhdarchids may well have been skilled at fast, level flight (as is
Rynchops), this lifestyle does not explain the probably proficient terrestrial abilities present in azhdarchids. More importantly, there is nothing in the azhdarchid skull showing that it was streamlined and laterally compressed as required for this hypothesis, nor is there any indication that the jaw joint or back of the skull was built to withstand jarring impacts, nor that the upper jaw could be elevated relative to water level, nor that the jaws were capable of rapid closure, as is the case in
Rynchops (Tomkins 1951, Bock 1960, 1964, Zusi 1962). The predictions are not fulfilled, so the skimming hypothesis is rejected.
Or, at least, the hypothesis that azhdarchids were
obligate skimmers is rejected. Tomkins (1963) wrote of his surprise on learning that Royal terns
Thalasseus maximus and Caspian terns
Hydroprogne caspia are both capable of skimming behaviour, even though they lack the many unusual features present in
Rynchops. Might azhdarchids, also, have been facultative skimmers? I would say that we can’t rule it out, but (1) there’s no evidence in its support and it’s therefore nothing more than a speculation, and (2) it’s still less well supported than other hypotheses.
We can also reject the rather similar Hypothesis 5: that azhdarchids were albatross-like surface gleaners, picking up prey from the water surface. The birds that do this are specialized for gliding and lack proficient terrestrial abilities, they have to have a flexible neck as they need to reach down and behind themselves as they pick up prey from the water surface, and they all have down-curved bill tips, presumably to aid in grabbing prey.
Finally, there’s Hypothesis 6: that azhdarchids were stork-like generalists, picking up assorted invertebrate and vertebrate prey from shallow water and/or terrestrial environments. So far as I can tell, this is the only hypothesis where all of the predictions are met. Azhdarchids have the proficient terrestrial abilities required for a stork-like lifestyle, and lack features indicating a dedicated aerial lifestyle. Their jaws are elongate but lack the specializations present in skimmers, mud-probers or surface gleaners, and their long, straight neck vertebrae indicate that they could only raise and lower the neck vertically. That’s ok for picking up animals from the ground and/or the water, but not much else. I therefore find Hypothesis 6 to be the only one that matches the evidence.
ConclusionsSo having completed this little exercise I still regard the skimming hypothesis as poorly founded and problematic, and I remain very much in favour of the stork hypothesis. It should be noted that azhdarchids lack the specializations seen in some stork taxa.
Mycteria (wood storks), for example, has a gently down-curved bill, a particularly dense array of Herbst corpuscles, and muscles that allow the jaws to be closed within 25 milliseconds (one of the fastest reflexes among vertebrates). These features are used by the birds as they search - using touch alone - for submerged prey (Hancock 1985).
Anastomus, the Open-billed stork, has scopate tomial edges (meaning that it possesses tiny brush-like structures along the margins of its bill) and upper and lower jaws that bow away from each other, meaning that their edges never meet. These are apparently specializations that assist in the holding of hard-shelled prey (Gosner 1993). Rather, azhdarchids seem most like the most generalized storks, such as the
Ciconia species. These eat everything from large insects, to frogs, fish, small crocodilians and mammals, and they patrol marshy areas and flooded meadows as well as dry grasslands for such prey. In fact they can make a living just about everywhere, and if you wanted to you could draw another parallel with azhdarchids here.
More research on this area is needed, but having said that I realize I’ve pretty much just written the better part of a paper on the subject. At some stage I’ll re-vamp it for publication… perhaps with Mark as co-author. And on that note, the illustration above is Mark’s, and I use it here with permission. It can be seen in its original context
here.
Yes yes, phd thesis, blah blah blah. It will be done by the end of this month, honest. For the latest news on Tetrapod Zoology do go
here.
Refs - -
Arthur, S. C. 1921. The feeding habits of the Black skimmer.
The Auk 38, 566-574.
Bennett, S. C. 2001. The osteology and functional morphology of the Late Cretaceous pterosaur
Pteranodon. Part II. Size and functional morphology.
Palaeontographica Abteilung A 260, 113-153.
Bock, W. J. 1960. Secondary articulation of the avian mandible.
The Auk 77, 19-55.
- . 1964. Kinetics of the avian skull.
Journal of Morphology 114, 1-42.
Frey, E., Buchy, M.-C. & Martill, D. M. 2003. Middle- and bottom-decker Cretaceous pterosaurs: unique designs in active flying vertebrates. In Buffetaut, E. & Mazin, J.-M. (eds)
Evolution and Palaeobiology of Pterosaurs. Geological Society Special Publication 217. The Geological Society of London, pp. 267-274.
Gerritsen, A. F. C. & Meijboom, A. 1986. The role of touch in prey density estimation by
Calidris alba.
Netherlands Journal of Zoology 36, 530-562.
Gosner, K. L. 1993. Scopate tomia: an adaptation for handling hard-shelled prey?
Wilson Bulletin 105, 316-324.
Halstead, B. 1989.
Dinosaurs and Prehistoric Life. Wm Collins Sons & Co., Glasgow.
Hancock, J. 1985. Storks and spoonbills. In Perrins, C. M. & Middleton, A. L. A. (eds)
The Encyclopedia of Birds. Guild Publishing (London), pp. 72-81.
Kellner, A. W. A. & Langston, W. 1996. Cranial remains of
Quetzalcoatlus (Pterosauria, Azhdarchidae) from Late Cretaceous sediments of Big Bend National Park, Texas.
Journal of Vertebrate Paleontology 16, 222-231.
Langston, W. 1981. Pterosaurs.
Scientific American 244 (2), 92-102.
Lawson, D. A. 1975. Pterosaur from the latest Cretaceous of west Texas: discovery of the largest flying creature.
Science 187, 947-948.
Lehman, T. M. & Langston, W. 1996. Habitat and behavior of
Quetzalcoatlus: paleoenvironmental reconstruction of the Javelina Formation (Upper Cretaceous), Big Bend National Park, Texas.
Journal of Vertebrate Paleontology 16 (Suppl. 3), 48A.
Nebel, S., Jackson, D. L. & Elner, R. W. 2005. Functional association of bill morphology and foraging behaviour in calidrid sandpipers.
Animal Biology 55, 235-243.
Nesov, L. A. 1984. Upper Cretaceous pterosaurs and birds from central Asia.
Paleontology Journal 1984 (1), 38-49.
Ősi, A., Weishampel, D. B. & Jianu, C. M. 2005. First evidence of azhdarchid pterosaurs from the Late Cretaceous of Hungary.
Acta Palaeontologica Polonica 50, 777-787.
Padian, K. 1988. The flight of pterosaurs.
Natural History 97 (12), 58-65.
Paul, G. S. 1987a. The science and art of restoring the life appearance of dinosaurs and their relatives - a rigorous how-to guide. In Czerkas, S. J. & Olson, E. C. (eds)
Dinosaurs Past and Present Vol. II. Natural History Museum of Los Angeles County/University of Washington Press (Seattle and London), pp. 4-49.
- . 1987b. Pterodactyl habits - real and radio-controlled.
Nature 328, 481.
Piersma, T., van Aelst, R., Kurk, K., Berkhoudt, H. & Maas, L. R. M. 1998. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics?
Proceedings of the Royal Society of London B 265, 1377-1383.
Tomkins, I. T. 1951. Method of feeding of the Black skimmer
Rynchops nigra.
The Auk 68, 236-239.
Tomkins, I. T. 1963. Skimmer-like behavior in the Royal and Caspian terns.
The Auk 80, 549.
Unwin, D. M., Bakhurina, N. N., Lockley, M. G., Manabe, M. & Lu, J. 1997. Pterosaurs from Asia.
Journal of the Paleontological Society of Korea, Special Publication 2, 43-65.
Wellnhofer, P. & Buffetaut, E. 1999. Pterosaur remains from the Cretaceous of Morocco.
Paläontologische Zeitschrift 73, 133-142.
Witmer, L. M. & Rose, K. D. 1991. Biomechanics of the jaw apparatus of the gigantic Eocene bird
Diatryma: implications for diet and mode of life.
Paleobiology 17, 95-120
Zusi, R. 1962. Structural adaptations of the head and neck in the black skimmer
Rynchops nigra Linneaus.
Publications of the Nuttall Ornithological Club 3, 1-101.
Labels: archosaurs, Mesozoic, pterosaurs









