Lunging is expensive, jaws can be noisy, and what’s with the asymmetry? Rorquals part III

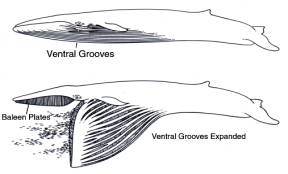
In the previous post I discussed the basic anatomy and behaviour involved in lunge-feeding, a style of predation practiced by rorquals, the biggest, fastest and most dynamic of baleen-bearing cetaceans. By engulfing literally tons of water within a unique, flexible buccal pouch, rorquals change shape from ‘a cigar shape to the shape of an elongated, bloated tadpole’ (Orton & Brodie 1987, p. 2898). Their feeding style is anything but passive: Paul Brodie, an expert on rorqual feeding, has described it as ‘the largest biomechanical action in the animal kingdom’. After discussing rorquals with whale expert Nicholas Pyenson, my good friend Matt Wedel, in one of several blog posts on rorquals and other mysticetes (that’s three separate links there), provided the most excellent quote…
The big baleen whales pick their targets and engulf them with their giant jaws and extensible mouth/throat region. They are often feeding on swarms of krill that measure kilometers in extent. Rather than think of big whales as filter feeders, we should think of them as predators that take bites off of superorganisms that are hundreds of times larger. The fact that the krill are strained out of the water by the baleen is a matter of processing - it comes after the whale has taken a bite.
That’s great: I’ll be stealing it for use in lectures. The photo at top features the immense jaws of an Antarctic blue whale, kept at Washington D.C.’s Garber Facility (part of the National Museum of Natural History), and is borrowed from here on Matt's blog site.
 Recent studies show that lunge-feeding is not just dynamic, it is also extremely expensive in metabolic terms, and even though rorquals glide as they lunge (thereby conserving some energy), it still seems that lunge-feeding is so energetically costly that constraints are imposed on rorqual behaviour. Theoretically, large-bodied species store more oxygen thanks to their size, and therefore have a higher theoretical aerobic dive limit (TADL). Indeed in marine mammals as a whole there is a trend of increasing dive depth and duration with increasing body size. If we look at the largest rorquals – the blue and fin – we find TADLs of 31.2 and 28.6 minutes. Yet the actual aerobic dive limits of the two species are respectively 7.8 and 6.3 minutes (Croll et al. 2001). For comparison, right whales – which weigh about half as much as blue whales – spend about twice as long foraging under water as blue whales. To quote Acevedo-Gutiérrez et al. (2002) ‘the largest predators on earth have the shortest dive durations relative to their TADL’ (p. 1747). Note also that rorquals don’t dive deep for their size: fin whales have been reported to dive down to 470 m, but that’s not deep for such a big animal (total length 18-25 m), nor were the dives in question long in duration at less than 13 minutes [adjacent photo, from the Right Whale Aerial Surveys site, shows a feeding Fin whale].
Recent studies show that lunge-feeding is not just dynamic, it is also extremely expensive in metabolic terms, and even though rorquals glide as they lunge (thereby conserving some energy), it still seems that lunge-feeding is so energetically costly that constraints are imposed on rorqual behaviour. Theoretically, large-bodied species store more oxygen thanks to their size, and therefore have a higher theoretical aerobic dive limit (TADL). Indeed in marine mammals as a whole there is a trend of increasing dive depth and duration with increasing body size. If we look at the largest rorquals – the blue and fin – we find TADLs of 31.2 and 28.6 minutes. Yet the actual aerobic dive limits of the two species are respectively 7.8 and 6.3 minutes (Croll et al. 2001). For comparison, right whales – which weigh about half as much as blue whales – spend about twice as long foraging under water as blue whales. To quote Acevedo-Gutiérrez et al. (2002) ‘the largest predators on earth have the shortest dive durations relative to their TADL’ (p. 1747). Note also that rorquals don’t dive deep for their size: fin whales have been reported to dive down to 470 m, but that’s not deep for such a big animal (total length 18-25 m), nor were the dives in question long in duration at less than 13 minutes [adjacent photo, from the Right Whale Aerial Surveys site, shows a feeding Fin whale].
Goldbogen et al. (2006) studied the kinematics of diving and lunge-feeding fin whales and showed that the rapid acceleration attained during lunge-feeding is immediately met by a relatively larger deceleration, presumably caused by the opening of the buccal pouch. The whales also rolled their bodies during lunging and may in fact spin about their long axis during feeding events, and at the bottom of a feeding dive a whale undertook a series of vertical excursions. It seems that the rapid acceleration and deceleration, and the dynamic movement, involved in lunge-feeding is highly costly, forcing rorquals to limit their dive time, and to increase the time that they need to spend at the surface recovering (Acevedo-Gutiérrez et al. 2002).
Some very interesting implications result from this expensive feeding style. Because lunge-feeding is so costly, it is likely only profitable where prey concentrations are high. Lunge-feeding rorquals cannot make a living anywhere there is suitable prey, therefore, but are ecologically tied to productive regions such as submarine canyons and the Southern Boundary of the Antarctic Circumpolar Current. A blue whale has been estimated to require one metric ton of krill per day.
When this is combined with the fact that some of the prey that rorquals depend upon, such as krill, are declining, it becomes clear why certain rorqual populations are struggling to recover from the days of commercial whaling. Indeed work on African hunting dogs Lycaon pictus has shown that high metabolic costs incurred during predation cause some species to be competitively inferior to others, forcing their populations to remain at low levels (Gorman et al. 1998). So lunge-feeding is a high-maintenance activity, and we should not be surprised that lunge-feeding rorquals that lunge-feed only on specific prey species are endangered, and liable to decline.
 Here it’s worth noting that different rorqual species specialize on different prey, though some (the minkes and the fin whale) seem to be opportunists. Sei whales specialize on crustaceans, in particular on copepods, and blue whales are specialist krill predators (Sigurjónsson 1995). Furthermore, not all rorqual species feed by lunging – the sei in particular uses a technique called skimming, whereby the whale keeps its mouth slightly open and moves forward through a body of prey at a continuous speed. It would be interesting to know how the morphology, kinematics and energetics of the sei compare to those of lunge-feeding rorquals, but so far as I know these issues remain largely unstudied. We do know that its baleen is particularly fine, allowing it to filter the comparatively small copepods [adjacent photo, also from the Right Whale Aerial Surveys site, shows a feeding sei. It’s feeding on its side. Hmm].
Here it’s worth noting that different rorqual species specialize on different prey, though some (the minkes and the fin whale) seem to be opportunists. Sei whales specialize on crustaceans, in particular on copepods, and blue whales are specialist krill predators (Sigurjónsson 1995). Furthermore, not all rorqual species feed by lunging – the sei in particular uses a technique called skimming, whereby the whale keeps its mouth slightly open and moves forward through a body of prey at a continuous speed. It would be interesting to know how the morphology, kinematics and energetics of the sei compare to those of lunge-feeding rorquals, but so far as I know these issues remain largely unstudied. We do know that its baleen is particularly fine, allowing it to filter the comparatively small copepods [adjacent photo, also from the Right Whale Aerial Surveys site, shows a feeding sei. It’s feeding on its side. Hmm].
Thanks to the work of August Pivorunas, Paul Brodie and colleagues, the engulfing mechanism of rorquals has been reasonably well understood since the 1970s. However, questions always remained. How is it that, during lunge feeding, agile, highly reactive prey remain within the mouth cavity prior to the mouth’s closure? Man-made devices of similar size are incapable of retaining prey without them escaping prior to the devices’ closure (Brodie 1978). When a rorqual carcass is processed at a whaling station, the soft tissue of the throat is removed by flensing. Using cables and straps, the jaws are then winched open, and the tendons and muscles holding the mandibles in place are then cut, freeing the jaw from the skull. Because the jaw is winched open without the very heavy throat tissue attached, its movement during the procedure approximates the natural movement of the jaw when the animal is alive and underwater. As the jaw is winched open ‘a familiar sequence of sounds was observed to originate from the jaw apparatus … a growl or rumble, a low hydraulic suction noise, following by a powerful knock, the latter seeming to emanate from the tip of the jaw’ (Brodie 1993, p. 546). The noise reverberated throughout the jaw, making the entire structure vibrate. Unusual loud noises have been reported from live, feeding fin whales, so what Brodie reported apparently occurs in live whales, and not just dead ones.
What might cause these noises? Could it be that the articular condyles of the jaw bones were grinding against the bones of the skull? Well, no, as large masses of collagen and lipid are sandwiched between the lower jaw and skull, and in the specimens Brodie examined there was no suggestion that this tissue had been compromised. Could it be that the jaw tips were grinding together? Again, no, as soft tissue separates the jaw tips and, anyway, the jaw tips were being forced apart when the noises were being made, not together. Brodie (1993) concluded that the noise was a consequence of the stretching apart of a synovial capsule located between the jaw tips. And, funnily enough, here we have something that is of direct relevance to all of us (well, most of us. Well, those of us who have heard our joints make crack noises).
As synovial capsules are forced apart, a partial vacuum forms in the joint cavity. Adjacent water vapour and blood gases from surrounding tissues rush to fill the vacuum, and as it collapses a noise results. Such noises range from low rumbles to loud knocks. This process is termed pseudocavitation (to distinguish it from cavitation: the process whereby the medium actually ruptures), and I’ve just realized that this solves one of the greatest mysteries in all of biomechanics: why our knuckles crack. I can’t tell you how many times I’ve sat around with colleagues, pondering this very question.
If the lower jaws of fin whales really do make a loud bang or crack when they are opened to full gape, we can speculate that the whales might use this to help them retain prey within the mouth during engulfment. Captured prey would be startled away from the jaw edges by the noises, and this isn’t unlikely given that we’ve long known that rorquals exploit the behavioural traits of their prey to concentrate them during predation (it is well known that humpbacks use bubbles to encircle prey, and in fact fin and Bryde’s whales have been reported doing this too). To my knowledge, the ‘noisy jaw’ hypothesis has only been proposed for fin whales. Is it unique to this species, or practiced more widely?
And speaking of fin whales…. generally speaking, tetrapods have symmetrical bodies and symmetrical arrangements of pigmentation. Why then are fin whales asymmetrical? Mostly dark on the left side of the head (this goes for the baleen and the left side of the tongue), they are mostly light on the right side (and, again, this goes for the baleen and the right side of the tongue). While individuals belonging to various species will sometimes exhibit asymmetrical pigmentation (and rorquals, such as minkes and sei whales, are among them), fin whales are consistently like this: all of them.
 Does this serve a function? Mostly it has been thought that it is something to do with counter-shading: if the whale swims anti-clockwise around its prey it might be camouflaged against the water and hence invisible, or is it that it swims clockwise around its prey, frightening them with its vivid whiteness and causing them to bunch up? Both ideas have been proposed (Ellis 1982). Most recently, cetologists seem to have favoured the idea that fin whales actually swim on their right side while lunge-feeding, thereby using a sort of rotated counter-shading. I’ve seen photos that apparently support this idea of right-sidedness, but I don’t know if there any good studies on the subject. There is widespread evidence for handedness across Tetrapoda (including in whales), so does this mean that all fin whales are right-handed, or left-handed?
Does this serve a function? Mostly it has been thought that it is something to do with counter-shading: if the whale swims anti-clockwise around its prey it might be camouflaged against the water and hence invisible, or is it that it swims clockwise around its prey, frightening them with its vivid whiteness and causing them to bunch up? Both ideas have been proposed (Ellis 1982). Most recently, cetologists seem to have favoured the idea that fin whales actually swim on their right side while lunge-feeding, thereby using a sort of rotated counter-shading. I’ve seen photos that apparently support this idea of right-sidedness, but I don’t know if there any good studies on the subject. There is widespread evidence for handedness across Tetrapoda (including in whales), so does this mean that all fin whales are right-handed, or left-handed?
That’s it on rorquals for now, though I plan at some stage to talk about the recently resurrected and recently discovered taxa, such as the Pygmy blue whale, Antarctic minke and Omura’s whale. And what is it with the name Balaenoptera musculus?
One last thing. I can’t go without relating the amazing tale of how I personally encountered Brodie’s 1993 paper ‘Noise generated by the jaw actions of feeding fin whales’. While collecting papers at
For the latest news on Tetrapod Zoology do go here.
Refs - -
Acevedo-Gutiérrez, A., Croll, D. A. & Tershy, B. R. 2002. High feeding costs limit dive time in the largest whales. The Journal of Experimental Biology 205, 1747-1753.
Brodie, P. F. 1978. Alternative sampling device for aquatic organisms. Journal of the Fisheries Research Board of
- . 1993. Noise generated by the jaw actions of feeding fin whales. Canadian Journal of Zoology 71, 2546-2550.
Croll, D. A., Acevedo-Gutierrez, A., & Tershy, B. R. & Urbán-Ramírez, J. 2001. The diving behavior of blue and fin whales: is dive duration shorter than expected based on oxygen stores? Comparative Biochemistry and Physiology 129A, 797-809.
Ellis, R. 1982. The Book of Whales. Alfred Knopf,
Goldbogen, J. A., Calambokidis, J., Shadwick, R. E., Oleson, E. M., McDonald, M. A. & Hildebrand, J. A. 2006. Kinematics of foraging dives and lunge-feeding in fin whales. The Journal of Experimental Biology 209, 1231-1244.
Gorman, M. L., Mills, M. G., Raath, J. P. & Speakman, J. R. 1998. High hunting costs make African wild dogs vulnerable to kleptoparasitism by hyaenas. Nature 391, 479-481.
Orton, L. S. & Brodie, P. F. 1987. Engulfing mechanisms of fin whales. Canadian Journal of Zoology 65, 2898-2907.
Sigurjónsson, J. 1995. On the life history and autecology of